AEHRC secures quality management certification for software-based medical devices
CSIRO’s Australian e-Health Research Centre (AEHRC) has nearly two decades of experience in creating and implementing digital health tools and devices.
We’re committed to the quality and compliance of the digital medical devices we develop on our own and in collaboration with our partners.
Early planning around regulatory requirements is crucial for optimising resources and ensuring health technology is market ready.
That’s why we’ve really outdone ourselves with our new ISO 13485:2016 certification.
What does ISO 13485:2016 certification mean?
Developing software for health and medical applications is complex, and all software as a medical device (SaMD) products must meet rigorous regulatory requirements.
Our certification demonstrates that we operate in compliance with international standards for the design, development, manufacturing, installation and servicing of software-based devices.
Collaborating with us can also accelerate the translation from concept to market. Our commitment to adhering to TGA and FDA requirements, along with our ISO 13485 certification, guarantee a robust QMS.
How do we ensure that all products meet the standards?
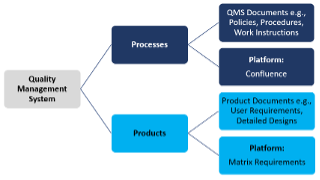
Our quality management system is comprised of two vital components – processes and products.
We follow standardised procedures that guide us through each stage of product development, from initial planning to final production.
We develop comprehensive technical files for our SaMD products that house everything needed to bring the product to life. It contains key information like user requirements, system specifications, intricate product architecture, detailed designs and risk management. The thorough documentation ensures that all products we have a hand in are not only high-quality but meet user expectations.
The Australian e-Health Research Centre (AEHRC) is CSIRO's digital health research program and a joint venture between CSIRO and the Queensland Government. The AEHRC works with state and federal health agencies, clinical research groups and health businesses around Australia.